Research Focus
The Group of Translational Neurology (GTN) was created in 2018 by the Visiting Professor Athanasios Lourbopoulos (Head of the Group) in collaboration with the Ass. Professor Iordanis Mourouzis, under the support of Professor Constantinos Pantos (Director of the Department).
The Group focuses on finding drugs and ways to promote regeneration and plasticity after stroke utilizing long-term (chronic) experimental stroke on a translational basis. We use the model of middle cerebral artery occlusion (MCAo) in mice along with an extensive neurobehavioral battery of appropriate tests. We study the animals in all phases after stroke (up to 6 months after the ischemic lesion) trying to discover approaches that confer long-term clinical benefits to the animals. In depth Microscopy, molecular and neuroimmunological methods are our mainstream tools to reveal the relevant underlying pathophysiology and molecular mechanisms of stroke and our applied approaches. Interdisciplinary collaborations (Chemistry, Informatics, Imaging) provide further support to our work.
Our current mainstream research projects are:
1. Understanding and improving the translationality of chronic experimental stroke
Ischemic stroke is one of the three most common causes of death and the most common cause of disability in industrialized countries. Despite significant research, more than 90% of stroke patients do not receive causal therapy. This shortcoming likely stems from the inability to translate well-understood, therapeutically exploitable concepts from the laboratory to the bedside. This is the so-called "translational roadblock" in stroke research (recently reviewed in detail, Lourbopoulos A et al, Front Neuroscience 2021, doi: 10.3389/fnins.2021.652403).
Based on the above, we created in Institute for Stroke and Dementia Research (ISD) in Munich and apply in our Lab in Athens a platform of "mouse Stroke-Unit (mSU)" care that maximizes the translational efficacy of our stroke model (Lourbopoulos A et al, JCBFM 2017, doi: 10.1177/0271678X16660986). Our work (Figure 1) has proved that any treatment can be considered protective only if it affects the primary brain ischemic lesion and the focal neurological deficits in stroked animals, regardless of the treatment’s effect on mortality and/or systemic stroke sequelae. The mSU concept is the basis to perform studies of large infarcts under highly clinically translational conditions (human Stroke-Unit) with maximized translational chances to the clinics.
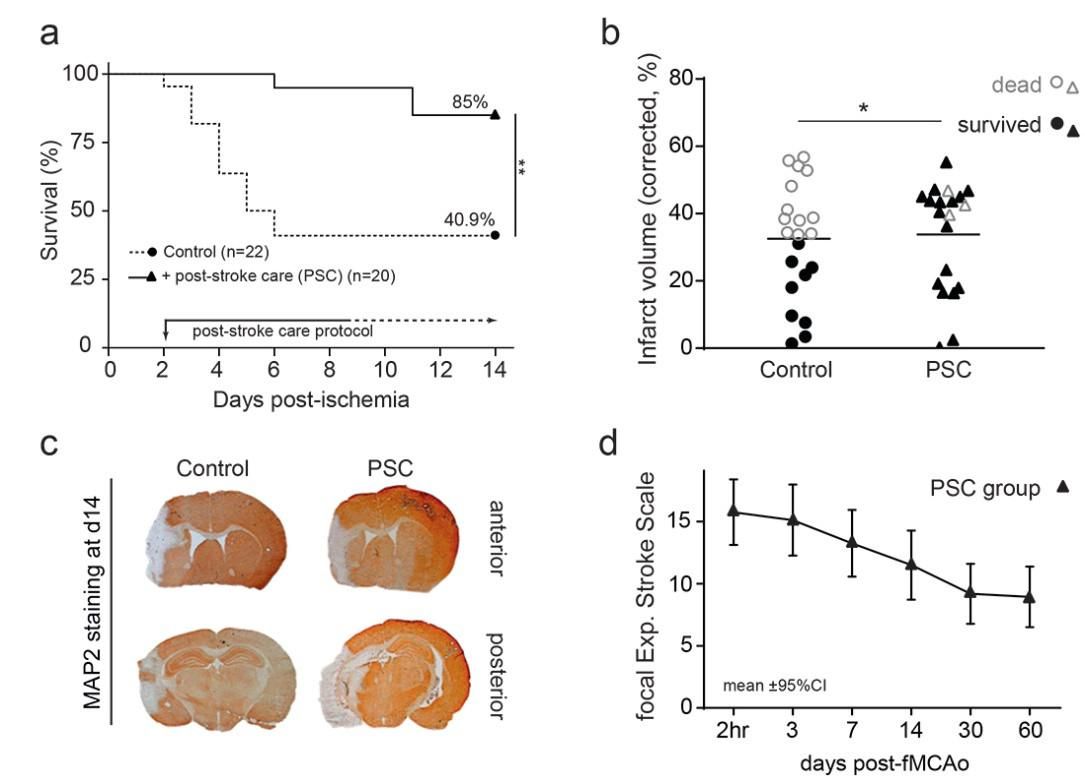
Figure 1. Post-stroke care (PSC) for mice reduces fMCAo mortality, without amelioration of main ischemic pathology.
Kaplan-Meier survival plot (a) shows that the appropriate post-stroke care (PSC group) doubled the 14-day survival rate compared to control mice. Importantly, it rescued animals with large infarcts (b) (infarct volume >35% of hemisphere, PSC group black filled triangles), without ameliorating their infarcts on day 14. Representative MAP2-stained brain sections (c), showing left hemispheric infarct (white tissue in the left hemisphere) and atrophy. Evidently, animals with large infarcts do not die when supported by PSC and have a chronic spontaneous recovery of approximately 40% (d) (focal Experimental Stroke Scale), which models the corresponding clinical status (78). The horizontal lines in b represent the mean values. *p<0.05; **p<0.01.
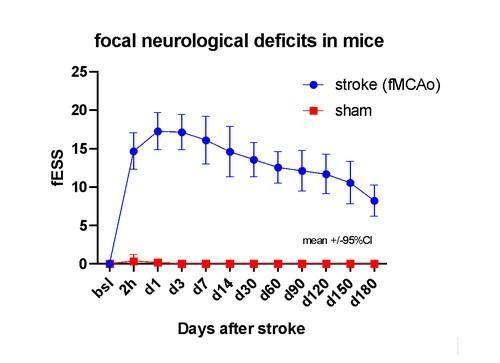
Recently we proved that the mSU concept can provide mice with large stroke and focal neurological deficits for ultra-long studies, as shown below.
Figure 2. The mSU concept allows for ultra-long study of severe focal neurological deficits in mice. The control (sham, no-stroke) animals show no deficits. The study was performed in a randomised, evaluator-blinded way until the end (6 months after stroke) of observation period. The neurological deficits were evaluated via the Experimental Stroke Scale (here its focal component, fESS).
2. Development of automated and unbiased tools for lesion analysis after stroke
Quantification of an ischemic stroke lesion in relevant rodent models is routinely based on laborious and time-consuming hand-based measurements on specially stained tissue sections. The most commonly used staining for stroke detection is the 2,3,5-Triphenyltetrazolium chloride (TTC) on thick brain sections, followed by Nissl or Cresyl Violet staining, MAP2-immunostaining or less common stainings (HSP72, HSP27) of thin brain slices. These manual measurements, despite their wide application and value, are highly subjective and prone to human error. At the same time, they do not provide any neuroanatomical information related to the lesioned areas in the brain.
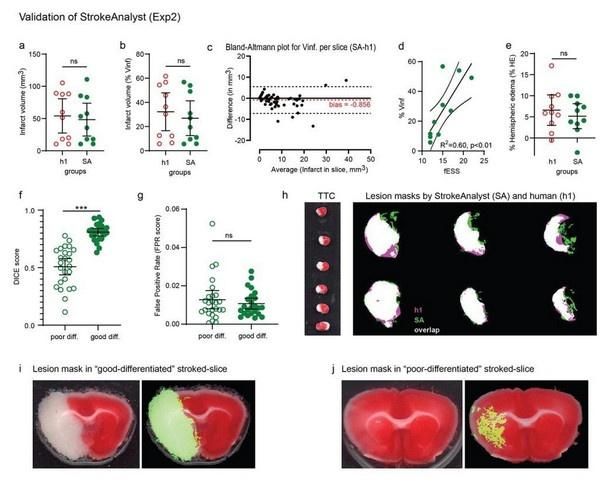
In collaboration with the Department of Electrical and Computer Engineering in the University of Patras, we developed and demonstrate a machine learning based system for stroke volumetry analysis and neuroanatomical mapping of stroke-lesions in TTC-stained sections. We call our system “StrokeAnalyst” (SA) (Damigos G et al, JCBFM 2022, doi: 10.1177/0271678X221083387). It detects fast, reliably, unbiased and automated the infarcted area (without false-positive inclusion of threshold-depended normal tissue), as shown below.
The StrokeAnalyst is currently under expansion and further development for histo-/immunohistological thin slices.
Figure 3. Results of SA on the validation experiment (Exp2) compared to manual volumetry. The infarct volumes measured by SA in mm3 (a) or as % percentage of left hemisphere (b) are similar to that of manual volumetry (h1). Bland-Altman plot for infarct volumes per single slices (c) shows an almost zero bias by SA compared to h1. Infarct volumes (as % Vinf) again correlate well to fESS values per animal (d). Hemispheric edemas as % percentage are also similar to human measurements (e). Dice-scores (f) and false positive rates (FPR, g) for SA- versus h1-masks, categorized per quality of TTC-staining (poor/good differentiation), show again that SA performs better in good differentiated TTC-slices. SA consistently has a very low FPR rate in both poor and good "differentiated" slices, indicating high specificity for infarct lesions. Representative overlay-projections (white) of SA (green) and h1 (pink) masks are shown in (h). Representative TTC-slices with good and poor color differentiation between infarcted and healthy tissue, with their corresponding SA lesion masks, are shown in (i) and (j) respectively.
3. Study of the actions of T3 as a therapeutic approach for microvascular dysfunction and multiorgan failure caused by sepsis.
In collaboration with the Group of Novel Pharmacological Approaches for Cardiovascular Disease we study the actions of T3 for microvascular dysfunction caused by sepsis. The study is performed in an in vivo model of ligation and perforation of the cecum in mice with the induction of peritonitis and is done in collaboration with the company Uni-Pharma Pharmaceutical Laboratories. The presence of tissue hypoxia in various organs is studied with an innovative method of immunohistochemistry based on pimonidazole. (Mourouzis I, Lourbopoulos A, Intensive Care Med Exp. 2021, doi: 10.1186/s40635-021-00382-y).
Contact Person #1
Contact Person #2